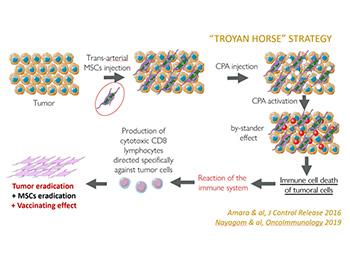
This so called « Gene-directed enzyme prodrug therapy (GDEPT) » consists in bringing an optimized gene - allowing to convert cyclophosphamide (CPA) into toxic metabolites 13 times more than native conversion - into the tumors, thanks to an efficient vector, mesenchymal stem cells (MSCs), and a specific (intra-arterial) administration. There, it eradicates the tumor and triggers a specific immune response, resulting in a vaccinating effect. POC in vivo have been established either in mice (treatment and rechallenge experiments), but also in an orthotopic model of hepatocarcinoma (VX2) in rabbits. Those last experiments confirmed breakthrough results, compared to Gold Standard (chemoembolization), either in terms of tumor & metastasis drastic reduction but also remission levels...
Gene-directed enzyme prodrug therapy - Cyclophosphamide highly active metabolite conversion - New cancer treatment - Gene & cell therapy
Erganeo se tient à votre écoute.